Thematic Axis : Joint diseases
Osteoarthritis (wear and tear on joint cartilage) is a major health problem because it is asymptomatic until it reaches an advanced stage, at which point no treatment is really effective, the only option being to fit an implant (arthroplasty). Despite international efforts to optimise materials, the in vivo lifespan of these implants is still very disappointing compared with that extrapolated from ex vivo simulations.
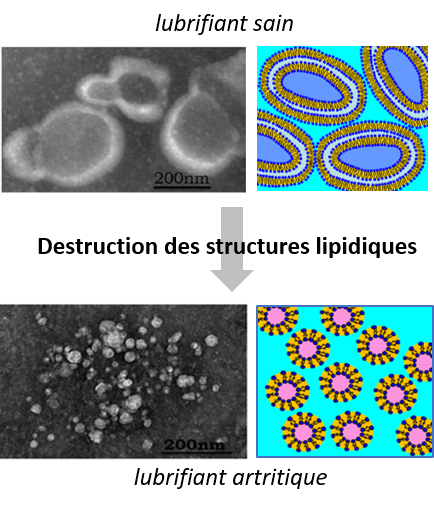
The discrepancies between in vivo and ex vivo lifetimes are mainly attributable to ex vivo test conditions that are insufficiently realistic given the mechanical and physico-chemical characteristics of biological lubricants. These biological lubricants are complex molecular media which, under tribological constraints, create molecular layers on the friction surfaces of joint implants, thereby modifying their friction and wear behaviour.
In this context, our initial joint work (LAMCOS / CARMEN / ILM, Constantin Ionut MATEI thesis, 2012, and Magdanena CONRECI, 2012) has shown that phospholipid-based molecular structures are essential in achieving a low coefficient of friction and low wear in a healthy joint. Consequently, the first objective of this area is to identify the phospholipid structures that play a key role in the development of osteoarthritis in order to use them as early markers in diagnosis.
The second objective is to master the mechanical and physicochemical interactions of these molecular assemblies with the surfaces of the joint implants and the adjacent tissues (bone/cartilage) in order to make a realistic estimate of the lifespan of the implants and to design therapeutic solutions that are less disabling than total arthroplasty.
Numerical models simulating the transmission of mechanical stress at multiple scales (from the organ: joint, to the cell: chondrocytes / osteocytes) will be used to optimise new therapeutic solutions to ensure that they are perfectly adapted to the patient from both a mechanical and biological point of view (sports, degenerative or inflammatory patients) and thus guarantee their duration in vivo.
The discrepancies between in vivo and ex vivo lifetimes are mainly attributable to ex vivo test conditions that are insufficiently realistic given the mechanical and physico-chemical characteristics of biological lubricants. These biological lubricants are complex molecular media which, under tribological constraints, create molecular layers on the friction surfaces of joint implants, thereby modifying their friction and wear behaviour.
In this context, our initial joint work (LAMCOS / CARMEN / ILM, Constantin Ionut MATEI thesis, 2012, and Magdanena CONRECI, 2012) has shown that phospholipid-based molecular structures are essential in achieving a low coefficient of friction and low wear in a healthy joint. Consequently, the first objective of this area is to identify the phospholipid structures that play a key role in the development of osteoarthritis in order to use them as early markers in diagnosis.
The second objective is to master the mechanical and physicochemical interactions of these molecular assemblies with the surfaces of the joint implants and the adjacent tissues (bone/cartilage) in order to make a realistic estimate of the lifespan of the implants and to design therapeutic solutions that are less disabling than total arthroplasty.
Numerical models simulating the transmission of mechanical stress at multiple scales (from the organ: joint, to the cell: chondrocytes / osteocytes) will be used to optimise new therapeutic solutions to ensure that they are perfectly adapted to the patient from both a mechanical and biological point of view (sports, degenerative or inflammatory patients) and thus guarantee their duration in vivo.
Contact : BERNOUD-HUBAC Nathalie, SFARGHIU Ana-Maria